Acute Leukemia
Evaluation of disease
Last update: October 1st, 2019
Flow cytometry has an essential role in the diagnosis and classification of acute leukemias working together with cytomorphology and cytochemistry on the detection and lineage assignment of blast cells in suspected samples, including the definition of acute leukemias of ambiguous lineage.
In the last years the flow cytometry based immunophenotyping has been increasing its role on the classification of the malignancy, detection of prognostic signatures and therapeutic targets and minimal residual disease evaluation.
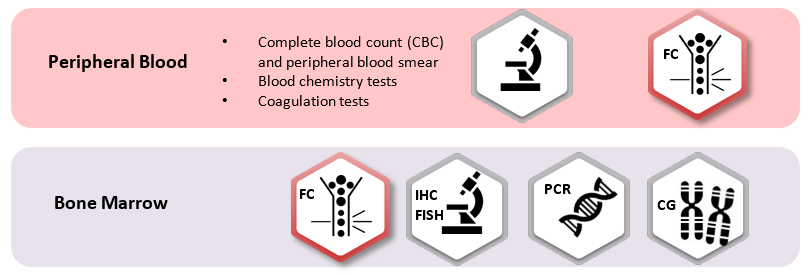
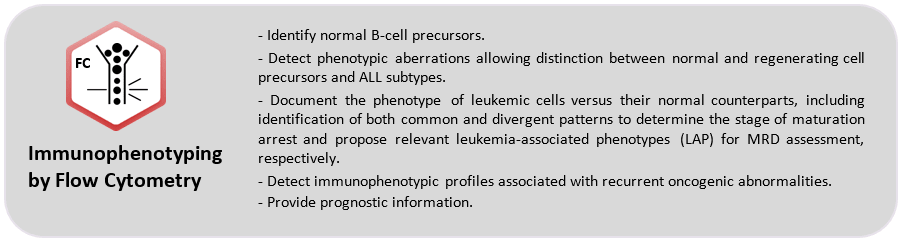
For the identification of immature B-, T- and NK-cells, and to distinguish malignant from normal ones, specific markers have been approved:
- CD45: differentiation of hematopoietic cells comes with a specific pattern of CD45lo intensity correlated with both lineage and maturation stage.
- CD34: it confirms the immature nature of the pathological population, expressed by a significant number of acute leukemias from any lineage.
- CyMPO: highly specific myeloid marker.
- CyCD3 and (Sm)CD3: specific T-lineage markers, interpreted both together in order to identify a CyCD3þ/SmCD3_/lo pattern, the most frequent phenotype of T-ALL.
- CD19 and CyCD79a: B-lineage markers, CD19 lacks specificity as it is also expressed in a subset of AML cases so CyCD79a can be used to B-lineage assignment.
- CD7: positive in virtually all cases of T-ALL and in a subset of, usually CyMPO-negative, AML.
Next Generation Flow™
In recent years, EuroFlow™ scientific consortium has developed a solution called Next Generation Flow in order to create and standardize fast, accurate and sensitive flow cytometry tests. Based on that knowledge, Cytognos offers a complete and scientific-based solution to cover all the needs for evaluation of acute leukemia patients.
Following NGF methodology, Cytognos offers the ALOT kit for the initial evaluation of bone marrow and peripheral blood with suspicion of acute leukemia, and orientation towards the Classification EuroFlow™ panels.
For classification of B-ALL, the Cytognos PBC-CORE contains the three backbone markers CD19, CD34 and CD45 that has proved to provide efficient positive identification of all malignant B-cell clones in every B-ALL patient. This combination together with the characterization markers provide an efficient differential diagnosis among the majority of B-ALL entities.
In order to analyze the obtained impressive and complex amount of data Cytognos offers the software Infinicyt™. Moreover, to standardize the analysis, Infinicyt 2.0 includes reference databases developed by EuroFlow™, including the so-called ALOT for the initial screening of acute leukemia.
Resources
Publications:
- van Dongen JJ, et al. EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia. 2012 Sep; 26(9):1908-75. Go to publication.
- Lhermitte L, et al. Automated database-guided expert-supervised orientation for immunophenotypic diagnosis and classification of acute leukemia. Leukemia. 2017 Apr; 2(4): 874-81. Go to publication.
- van Dongen J, et al. Minimal residual disease diagnostics in acute lymphoblastic leukemia: need for sensitive, fast, and standardized technologies. Blood. 2015 Jun; 125(26): 3996-4009. Go to publication.
- Theunissen P, et al. Standardized flow cytometry for highly sensitive MRD measurements in B-cell acute lymphoblastic leukemia. Blood. 2016 Jun; 129(3): 347-57. Go to publication.
- Arber DA, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016 May; 127(20): 2391-405. Go to publication.
- Lhermitte L, et al. EuroFlow Consortium. Automated identification of leukocyte subsets improves standardization of database-guided expert-supervised diagnostic orientation in acute leukemia: a EuroFlow study. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2021 Jan:34(1), 59–69. Go to publication.




