Plasma Cell Dyscrasias
Evaluation of disease
Last update: October 1st, 2019
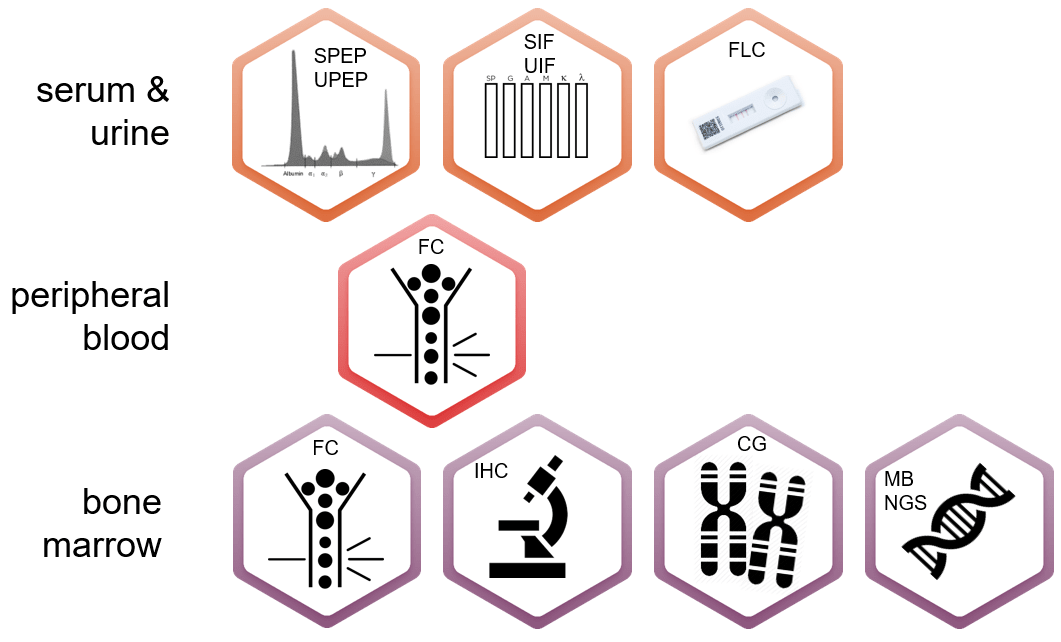
Multidisciplinary techniques should be used to characterize plasma cell dyscrasias, evaluate clonality and monitor the different disease stages. Evaluation techniques includes:
- Protein electrophoresis (SPEP or UPEP) measures M protein secreted by myeloma cells;
- Blood tests evaluate the levels of serum albumin and serum beta-2 microglobulin, kidney function, calcium levels and anemia. Beta 2- microglobulin is considered important for prognosis.
- Immunoglobulin quantitation measures IgG, IgA and IgM which are usually decreased in myeloma patients
- Assessment of free light chains (FLC) measures the excess of light chains found in myeloma patients.
- Other relevant methods are imaging techniques which can indicate how many can be found in the body (also includes size measurement). These techniques are IX-ray, MRI (Magnetic Resonance Imaging), CT scan (Computed Tomography), PET scan (Positron Emission Tomography).
- Bone marrow biopsy and aspirations allow a more in depth study of the PCD. cytogenetic, molecular and cellular-based techniques are used:
- Cytogenetic: fluorescence in situ hybridization (FISH) is used to determine standard and high-risk patients.
- Molecular: Evaluation of the specific gene translocations help define the number of clones and also to define risk groups.
- Cellular: Flow cytometry (immunophenotyping) has had an increased role in PCD evaluation and when used at diagnosis, is useful to assess clonality, abnormal plasma cell immunophenotype and prognosis. In recent years due to improvement of equipment and methodologies, flow cytometry is now paramount for the monitoring of minimal residual disease of PCD patients.

Immunophenotype of plasma cells
Identification of plasma cells by flow cytometry provides relevant information regarding diagnosis and classification, as well as prognostic stratification and minimal residual disease (MRD). The sample used for this methodology is bone marrow. Most recently, a newer strategy (CTPC evaluation) has been proposed to better identify patients with MGUS and SMM with high risk of progression to MM. Also, this strategy could be used in previously diagnosed MM patients to identify the ones with higher disease stability. The sample to be used in this new approach is peripheral blood that, although a bit less sensitive than bone marrow, is less invasive and allows for more frequent control of patient evolution.
Independent of the time point where flow cytometry is used during patient evaluation, some markers have been defined in consensus to be the most informative and relevant for assessment (note that the below descriptions are based mainly on bone marrow expected expressions):
- CD38 is present in B cell precursors and in germinal center B cells among others. CD38 is expressed brightly in normal and abnormal plasma cells but since new treatments against the CD38 molecules are being introduced, the expression of this marker by flow cytometry has become more difficult.
- CD138 is highly expressed in the later stage of B cell maturation, especially in both abnormal and normal plasma cells. CD138 expression can be affected in samples exposed to sodium heparin and aged samples. Therefore, it is recommended to process the samples using EDTA anticoagulant within the first 24-36 h.
- CD45 is expressed at variable levels in all leukocyte subsets. Normal plasma cells are usually positive for CD45. In samples with MGUS and MM, the expression can change to positive dim or negative.
- CD19 is expressed in all stages of B cell maturation, from pro-B to plasma cells, but it can be negative in different subsets of plasma cells, (around 30%). In abnormal cells CD19 is usually negative.
- CD56 is mainly expressed in NK cells. It is one of the most valuable markers to identify abnormal plasma cells in the bone marrow, as it is very frequently positive (around 86% of cases).
- CD27 is a memory marker expressed in germinal center, memory B and plasma cells. Usually a decrease in its expression in abnormal plasma cells is related with worse prognosis.
- CD117 is mainly expressed at different intensities in myeloid precursors and is highly expressed in mast cells. Normal plasma cells do not express this marker, but it is expressed in around one third of patient in which it is related with good prognosis.
- CD81 is expressed in hematopoietic cells at different levels. It is highly expressed in B cell precursors and is heterogeneous is normal plasma cells. Its expression in abnormal plasma cells is usually associated with worse prognosis.
- Kappa and Lambda – There are five types of heavy chains (G, A, D, E y M) to which the light chains Kappa or Lambda can be connected. Only a single combination of heavy chain and light chains can be found in each immunoglobulin. In patients not suffering from MM, it is possible to find all sorts of combinations between heavy and light chains, and this is, therefore, classified as polyclonal immunoglobulins. Kappa and Lambda markers are therefore, paramount in the confirmation of clonality of suspected abnormal plasma cells.
- β2-microglobulin – The expression of this marker has been shown to be a good prognostic marker when found in abnormal plasma cells.
Resources
Publications:
- Soh KT et al. Diagnosis of Plasma Cell Dyscrasias and Monitoring of Minimal Residual Disease by Multiparametric Flow Cytometry. Clin Lab Med. 2017 Dec;37(4):821-53. Go to publication.
- Rajkumar SV. Multiple myeloma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016 Jul;91(7):719–734. Go to publication.
- Flores-Montero, et al. Immunophenotype of normal vs. myeloma plasma cells: Toward antibody panel specifications for MRD detection in multiple myeloma. Cytometry B Clin Cytom. 2016 Jan;90(1):61-72. Go to publication.
- Jelinek T, et al. Current applications of multiparameter flow cytometry in plasma cell disorders. Blood Cancer Journal. 2017;7:e617. Go to publication.
- Paiva B, van Dongen JJM and Orfao A. New criteria for response assessment: role of minimal residual disease in multiple myeloma. Blood. 2015 May; 125(20):3059-68. Go to publication.
- Pérez-Andrés M, et al. Clonal plasma cells from monoclonal gammopathy of undetermined significance, multiple myeloma and plasma cell leukemia show different expression profiles of molecules involved in the interaction with the immunological bone marrow microenvironment. Leukemia.2005 Mar; 19(3):449-55. Go to publication.




