
Webinar – Experience with MM MRD in our clinical laboratory: theory and practice – 9 AM GMT
March 30, 2021 @ 11:00 - 12:00 UTC+1
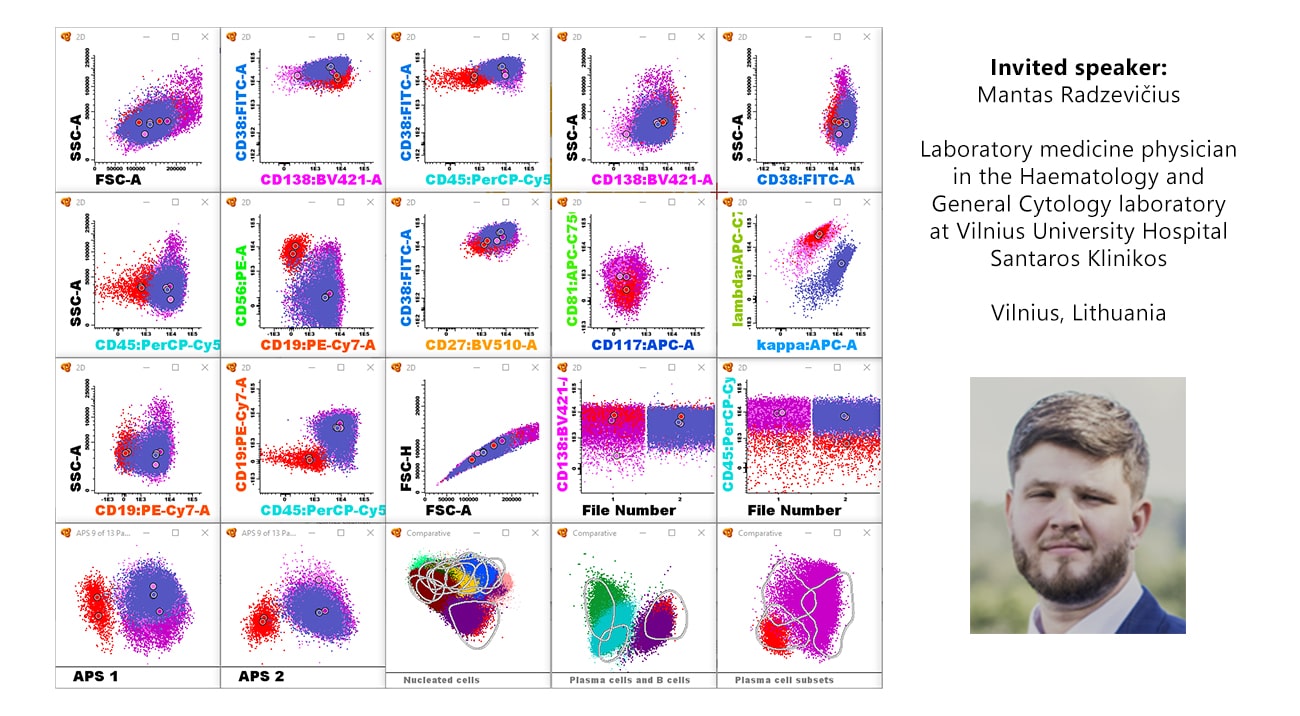
The level of minimal residual disease (MRD) is paramount to link the depth of response and long-term outcome. Recent advances in treatment demand a more sensitive response criteria which includes MRD evaluation. Therefore, MRD has been defined as one of the best surrogate markers to predict overall survival and accelerate the approval of new therapies in Multiple Myeloma (MM).
In this webinar, Mantas Radzevičius will explain:
- Theoretical basis and clinical use of MRD evaluation
- How to implement standard guidelines in a clinical flow cytometry laboratory
- MM MRD sample requirements and how to determine sample adequacy
- MM MRD consensus antibody panel
- Manual and automatic analysis – pitfalls and difficulties in analysis
- MM MRD report – relevant clinical information
Mantas Radzevičius works at Vilnius University Hospital Santaros Klinikos, in the Hematology and General Cytology laboratory, as a laboratory medicine physician. He performs flow cytometry assays in patients with hematological conditions including leukemia/lymphoma, multiple myeloma, PNH and MDS. Mantas cooperates with international organizations such as ESCCA and NOPHO. His PhD project is focused on the interaction between tumour cells and their immune microenvironment, as well as expression of immune checkpoint molecules.
11 AM GMT+2 (Madrid, Spain)
12 AM GMT+3 (Vilnius, Lithuania)
5 PM GMT+8 (Beijing, China)
8 PM GMT +11 (Sydney, Australia)
6 AM GMT-3 (Rio de Janeiro, Brasil)
5 AM GMT-4 (New York, NY, USA)
2 AM GMT-7 (Los Angeles, CA, USA)


